MedComm-Future Medicine | Neoadjuvant Strategies for Triple Negative Breast Cancer: Current Evidence and Future Perspectives

Open the phone and scan
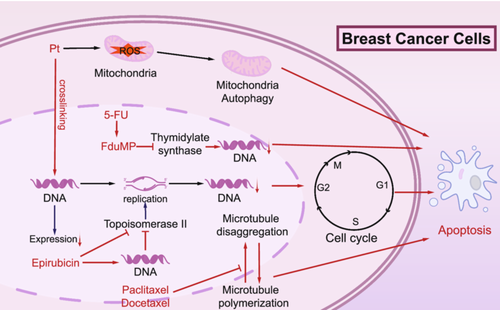
Mechanistic study of paclitaxel and other chemotherapeutic agents in neoadjuvant therapy for triple-negative breast cancer. This diagram illustrates the mechanisms of action of key chemotherapeutic agents targeting breast cancer cells. The agents affect various stages of the cell cycle and DNA processes to induce apoptosis. Carboplatin and cisplatin crosslink DNA strands, disrupting replication and transcription. Epirubicin intercalates into DNA and inhibits topoisomerase II, preventing proper DNA replication. 5-FU (5-fluorouracil) inhibits thymidylate synthase, depleting the nucleotide pool required for DNA synthesis. Paclitaxel and docetaxel stabilize microtubules, blocking their depolymerization, thereby arresting the cell cycle in mitosis. These combined effects lead to cell cycle arrest and apoptosis, effectively reducing tumor growth. 5-FU, 5-fluorouracil; DNA, deoxyribonucleic acid; G1, Gap 1 phase; G2, Gap 2 phase; M, mitosis phase; S, synthesis phase; thymidylate synthase, an enzyme essential for DNA synthesis; Topoisomerase II, DNA Topoisomerase II.
Triple-negative breast cancer (TNBC) is a highly aggressive subtype of breast cancer, characterized by poor prognosis and limited therapeutic options. Although neoadjuvant chemotherapy (NACT) remains the established treatment approach, its suboptimal efficacy associated with TNBC highlight the urgent need for optimized treatment strategies to improve pathological complete response (pCR) rates. This review provides a comprehensive overview of recent advancements in neoadjuvant treatment for TNBC, emphasizing pivotal breakthroughs in therapeutic strategies and the ongoing pursuit of innovative approaches to enhance precision medicine. It emphasizes the clinical value of platinum-based agents, such as carboplatin and cisplatin, which have shown significant improvements in pCR rates, particularly in TNBC patients with BRCA mutations. Additionally, the review explores progress in targeted therapies, including PARP inhibitors, AKT inhibitors, and Antiangiogenic agents, showcasing their potential for personalized treatment approaches. The integration of immunotherapy, particularly immune checkpoint inhibitor like pembrolizumab and atezolizumab, with chemotherapy has demonstrated substantial efficacy in high-risk TNBC cases. Future research priorities include refining biomarker-driven strategies, optimizing therapeutic combinations, developing antibody-drug conjugates (ADCs) targeting TROP2 and other biomarkers, and reducing treatment-related toxicity to develop safer and highly personalized neoadjuvant therapies. Furthermore, artificial intelligence has also emerged as a transformative tool in predicting treatment response and optimizing therapeutic decision-making in TNBC. These advancements aim to improve long-term outcomes and quality of life for patients with TNBC.
Article Access: https://doi.org/10.1002/mef2.70013
More about MedComm-Future Medicine: https://onlinelibrary.wiley.com/journal/27696456
Looking forward to your contributions.


