MedComm-Oncology | Epigenetic remodeling under oxidative stress: Mechanisms driving tumor metastasis

Open the phone and scan
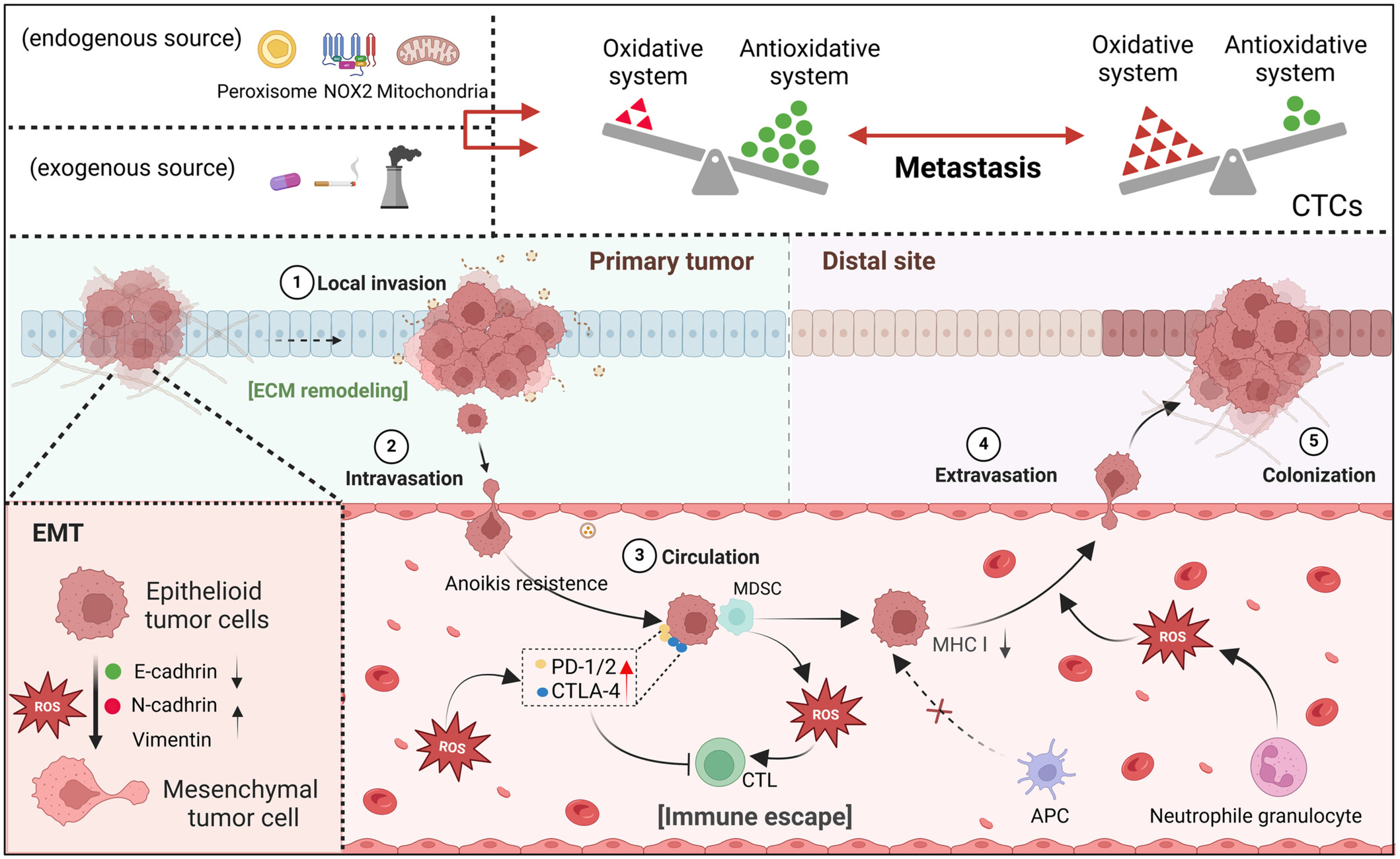
Reactive oxygen species (ROS) participate in the whole process of tumor metastasis. During metastasis, ROS are always at abnormal levels due to the dynamic imbalance of the redox system of tumor cells, coupled with some endogenous and exogenous initiating factors, such as mitochondrial damage and exposure to polluted air. Indeed, ROS are indispensable for the advancement of metastasis. Before metastasis, ROS can induce epithelial-mesenchymal transformation in tumor cells. In early metastasis, ROS promote the secretion of matrix metalloproteinases to remodel the extracellular matrix, which helps the local invasion of tumor cells and infiltration into the circulating system during circulation, while circulating tumor cells circumvent immunological surveillance and clearance by downregulating surface antigens, such as the class I major histocompatibility complex (MHC Ⅰ), and mediating the function of immunosuppressive cells. Additionally, ROS resulting from neutrophil granulocytes and other sources are involved in extravasation and colonization at secondary sites. APC, antigen presenting cells; CTLs, cytotoxic T lymphocytes; MDSC, myeloid-derived suppressor cells; NOX2, NADPH oxidase 2.
Tumor metastasis is a multistep, inefficient process orchestrated by diverse signaling pathways. Compared to primary tumor cells, disseminated tumor cells inevitably encounter higher oxidative stress in foreign environments. The levels of reactive oxygen species (ROS) fluctuate dynamically during different metastatic stages, adding complexity to the regulation of metastatic progression. Numerous studies suggest that epigenetic remodeling, a key reversible mechanism of gene regulation, plays a critical role in responding to oxidative stress and controlling gene expression profiles that drive metastasis. Despite extensive research, a comprehensive understanding of how oxidative stress impacts metastasis through epigenetic modifications remains elusive, such as DNA methylation, histone modification, ncRNAs, and m6A modification. Epigenetic therapeutic strategies, such as DNMT inhibitors, HDAC inhibitors (HDACis), and miRNA mimics, have shown promise, yet challenges related to immunogenicity, specificity, and delivery also exist. Furthermore, due to limited understanding, some drugs targeting m6A modification have yet to be explored. In this review, we provided an overview of how oxidative stress influences tumor metastatic behavior, summarized the epigenetic mechanisms involved in these processes, and reviewed the latest advancements in epigenetic-targeted therapies, which may pave the way to develop novel strategy for preventing or treating tumor metastasis.
Article Access: https://doi.org/10.1002/mog2.70000
More about MedComm-Oncology: https://onlinelibrary.wiley.com/journal/27696448
Looking forward to your contributions.


