MedComm-Oncology | cGAS-STING signaling in cancer: Regulation and therapeutic targeting

Open the phone and scan
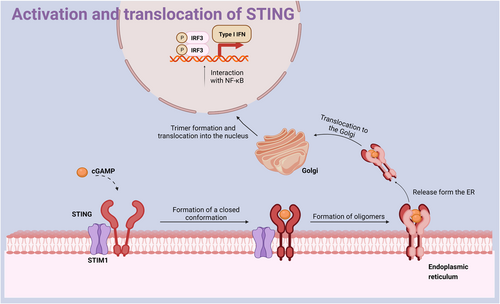
The activation and translocation of STING. STING interacts with STIM1 and localizes on the ER. When STING is not yet activated, it is in a dimeric state with an open conformation. When STING is activated by cGAMP, it changes to a closed conformation and forms dimers, tetramers, and even oligomers, which are released from the ER and translocated to the Golgi apparatus. cGAMP, cyclic guanosine–adenosine; ER, endoplasmic reticulum; STIM1, stromal interaction molecule 1; STING, stimulators of interferon genes.
Immunotherapy has revolutionized antitumor therapy. Since the discovery of stimulators of interferon genes (STING), efforts have been made to elucidate their mechanisms and physiological functions and explore the potential of STING as a therapeutic target in immune-related diseases and malignant tumors. In recent years, STING agonists have become a popular research topic. Activation of the cyclic GMP–AMP synthase (cGAS)-STING pathway produces large amounts of type I interferons, which play key roles in activating innate and acquired immune responses. The cGAS-STING pathway influences almost all aspects of tumorigenesis and has great antitumor potential. In addition, the activation of the cGAS-STING pathway is associated with tumor regression, prolonged survival of patients with cancer, and enhanced immunotherapy. Given the positive role of STING in antitumor immunity, the development of STING-targeted drugs is important. In this review, we summarize the activation and potential mechanisms of the cGAS-STING pathway, discuss the association of the cGAS-STING pathway with tumors and autoimmune diseases, and highlight research progress, clinical applications, and combination drug strategies for STING agonists.
Article Access: https://doi.org/10.1002/mog2.49
More about MedComm-Oncology: https://onlinelibrary.wiley.com/journal/27696448
Looking forward to your contributions.


