Molecular Biomedicine| Cancer metabolism and intervention therapy

Open the phone and scan
Metabolic reprogramming with heterogeneity is a hallmark of cancer and is at the basis of malignant behaviors. It supports the proliferation and metastasis of tumor cells according to the low nutrition and hypoxic microenvironment. Herein, a comprehensive understanding of the mechanisms of metabolic reprogramming will contribute to developing better anti-cancer strategies. Researchers from Chongqing University Cancer Hospital published a review in Molecular Biomedicine: Cancer metabolism and intervention therapy. This review systematically analyzes the characteristics of tumor metabolism from the perspectives of nutrient intake, biosynthesis, energy metabolism, redox metabolism, and tumor metabolites reshaping the tumor microenvironment. It also discusses some intervention strategies that target tumor metabolism.

Tumors reprogram multiple pathways associated with nutrient acquisition and metabolism to fulfill the biosynthetic, bioenergetic, and redox demands of malignant cells. Compared with normal cells, which rely primarily on oxidative phosphorylation (OXPHOS) to generate the energy needed for cellular processes, most tumor cells show significantly enhanced anabolism pathway, including aerobic glycolysis, glutaminolysis, fatty acid synthesis (FAS), and pentose phosphate pathway (PPP).
Cancer cells must balance biomass- and energy-producing processes to adapt to the challenging environments, including hypoxia and nutrient deprivation. In order to cope with the stress of survival: such as hypoxia, nutritional deficiencies, radiotherapy and chemotherapy, and anoikis, tumor cells must convert biosynthetic pathways into catabolic pathways, including autophagy, fatty acid oxidation and oxidative phosphorylation, to satisfy energy Required. Additionally, redox balance is essential for maintaining tumor cell survival and activity. Tumor cells produce NADPH through the Keap1/Nrf2 antioxidant system and PPP pathway to maintain the intracellular redox balance, promote tumor progression and treatment tolerance.
Tumor microenvironment (TME) refers to the area surrounding the tumor that is comprised of stromal, immune, and malignant cells, tumor vasculature, and sometimes adipocytes, and the exact composition of each stroma varies depending on cancer and tissue types (Fig.1). Malignant cells adapt TME through symbiotic metabolic interactions with other neighboring cells, including metabolic coupling, nutrient competition, and secreted metabolites as signaling molecules.
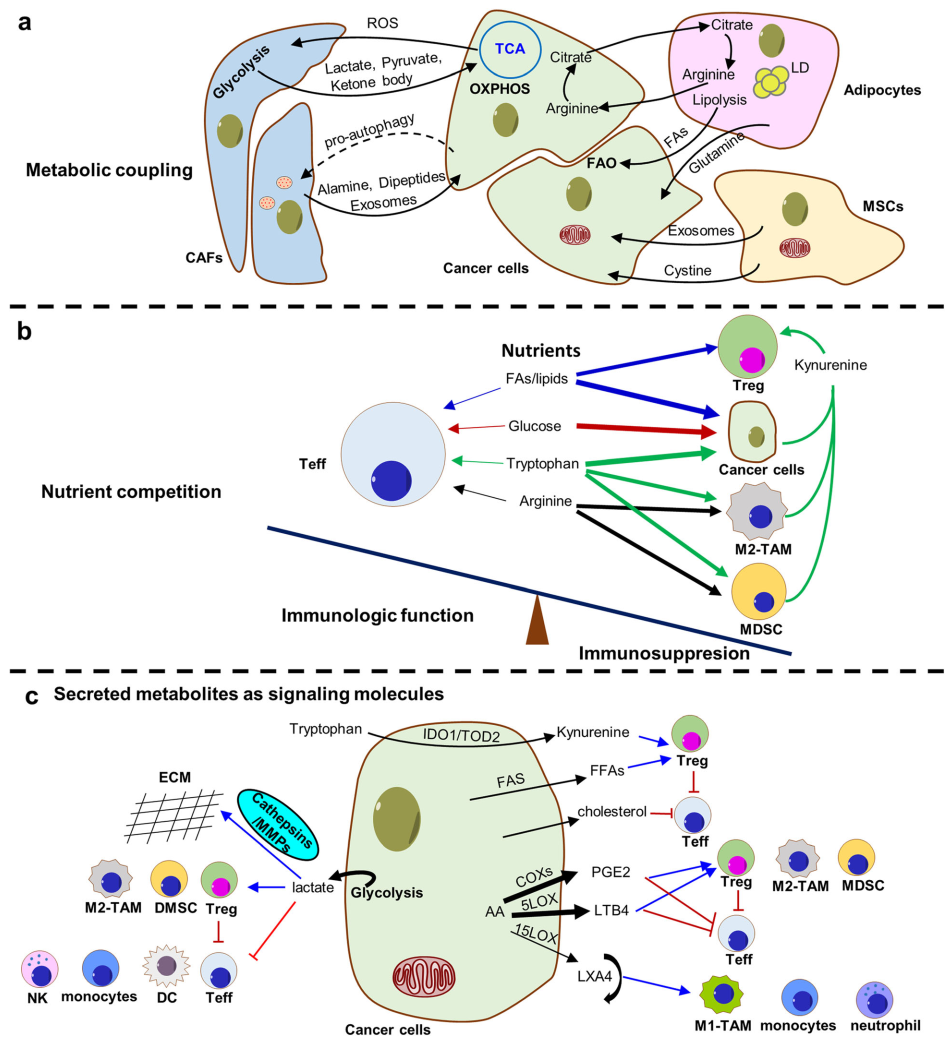
Fig.1 Metabolic interactions in the TME
However, there are still many limitations of this metabolic intervention strategy, such as chemotherapy resistance and severe side effects, due to metabolic heterogeneity in tumors, the plasticity of metabolic pathways.In this section, they highlight the potential approach of metabolic targets for cancer therapies, including combination of inhibitors targeting different metabolic pathways, induction of timed metabolic collapse, metabolic editing of CAR T-cells and correction of the mutation of metabolic enzymes by CRISPR/Cas9.
In summary, this article systematically analyzes the characteristics of tumor metabolism and discusses intervention strategies that target tumor metabolism. Considering that the heterogeneity of tumor metabolism is much smaller than the heterogeneity of tumor genes, anti-cancer strategies that target tumor metabolism have important clinical significance and prospects.
Article Access:https://link.springer.com/article/10.1186/s43556-020-00012-1
Website for Molecular Biomedicine: https://www.springer.com/journal/43556
Looking forward to your contributions


