Molecular Biomedicine | GEF-independent Ran activation shifts a fraction of the protein to the cytoplasm and promotes cell proliferation

Open the phone and scan
Ran (Ras-related nuclear) protein is a member of the Ras superfamily of small GTPases. Like other GTPases, Ran switches between GDP-bound (inactive) and GTP-bound (active) states. Multiple missense mutations in the C-terminus of Ran occur in cancers, but their biological significance remains unclear. Herein, researchers from Sichuan University reveal a new route of Ran activation independent of guanine nucleotide exchange factor (GEF), which may account for the hyper-proliferation induced by Ran cancer mutations.
Ran is overexpressed in a few cancers, and its activation - which in this work refers to being more GTP-bound rather than enzymatically active - is involved in cell proliferation, metastasis, and cellular transformation. Unlike other Ras superfamily proteins, Ran contains a unique C-terminal tail that packs against its G-domain, probably accounting for its ten-fold lower affinity for GTP compared with GDP. In order to study the function of C-terminal mutations, designed four C-dis mutants (A133D, L182A, M189D, and Y197A) to disrupt C-tail binding to the G domain. They discovered C-dis mutants showed higher relative affinities towards binding GTP, and were able to interact with effector proteins.
Next, author confirmed that the C-dis mutants were more charged with GTP and more cytoplasmic in human cells, and supported nuclear transport with slightly reduced efficiency.
Since Ran activation was previously shown to play a role in cellular transformation, the effects of Ran C-terminus cancer mutants were investigated. Several mild C-dis Ran cancer mutations were discovered. Cellular localization data showed that except for H30Y and P184S, the other cancer mutants were more localized to the cytoplasm compared to WT.
To investigate the underlying mechanism, they next analyzed several proteins that were reported to be perturbed by Ran overexpression or activation. The western analysis agreed well with the cell proliferation data(Fig 1.), suggesting that RanA183T promoted cell proliferation in HeLa and A549 cells, possibly through one or more of the pathways analyzed above.
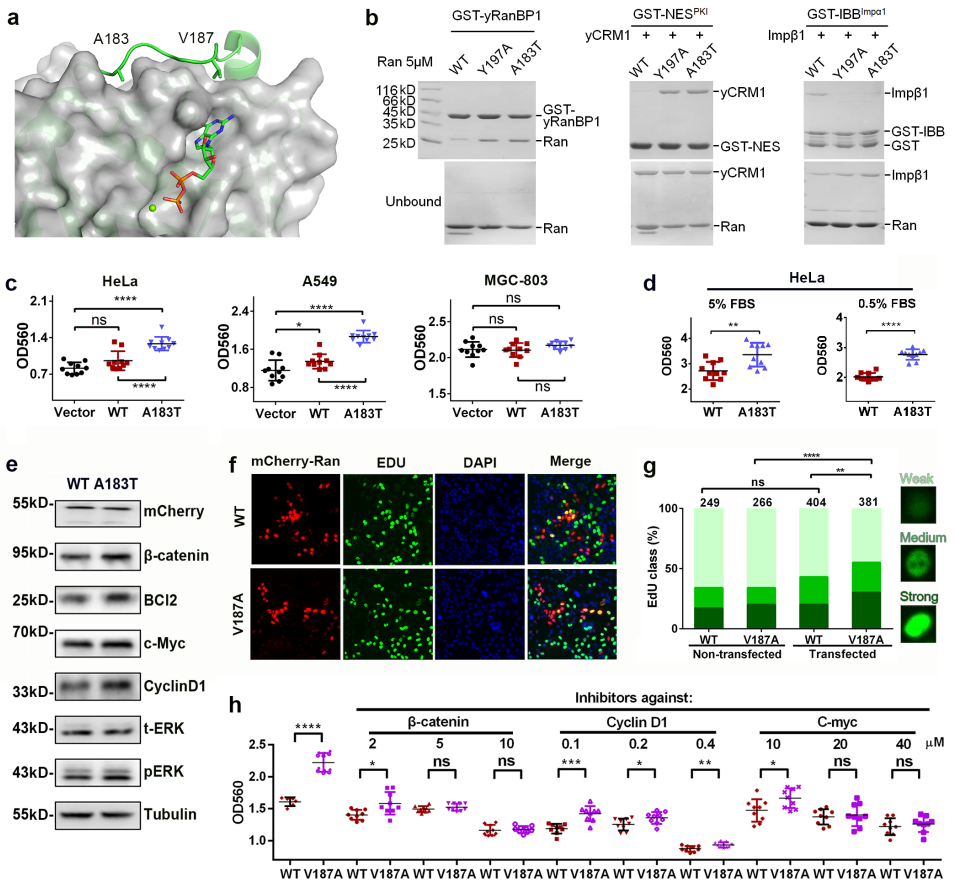
Fig 1. Enhanced cell proliferation by RanA183T and RanV187A
Thus, their work reveals a new route of Ran activation independent of guanine nucleotide exchange factor (GEF). It has important guiding significance for explaining the excessive proliferation caused by Ran cancer mutation.
Article Access: https://doi.org/10.1186/s43556-020-00011-2
Website for Molecular Biomedicine: https://www.springer.com/journal/43556
Looking forward to your contributions


