MedComm | A cyclic peptide retards the proliferation of DU145 prostate cancer cells in vitro and in vivo through inhibition of FGFR2

Open the phone and scan
As a group of RTKs family, fibroblast growth factor receptors (FGFRs) play critical roles in many key behaviors, including cell proliferation, migration, differentiation, and survival. As their ligands, FGFs including FGF1 (acidic FGF), FGF2 (basic FGF), FGF3-6, FGF7, FGF8-10, and FGF16-23 bind to different FGFRs and activate their corresponding receptors. However, altered FGFs/FGFRs signaling could result in pathogenesis. Usually, the FGF/FGFR signaling was amplified in various malignancies. For instance, Deng et al demonstrated that nearly half of gastric cancers demonstrated amplified FGFR2 signaling. Chen et al found that FGFR2 expression level and ligand-induced phosphorylation are responsible for the progression and poor prognosis in various cancers. These highlighted FGFR2 signaling as a potential cancer therapeutic target.

Although several chemicals have been designed against FGFR2, they did not exhibit enough specificity and might bring potential accumulated toxicity. Peptide-based drugs are catching more and more attention for their unique molecular properties. They showed lower immunogenicity but similar specificity when compared with the full-length protein. However, peptides are demonstrated greater selectivity than small chemical molecules. Moreover, peptides could be degraded into amino acids in vivo, which are totally nontoxic. These properties allow peptides to be excellent candidates for cancer-targeted therapy, such as FGFR2 inhibitors. As a drawback, the stability of peptides is still needs improvement. Cyclization is one of the major strategies to promote the stability of peptides. It has been employed to upgrade many instable peptides, and thus, cyclic peptides are attracting considerable interest owing to their efficiency in maintaining biostability within the proper range.
In this study, the authors developed an epitope peptide (P5) and its cyclic derivative (DcP5) based on the structure of FGF2 to limit the activation of FGFR2. The anticancer activities of P5 and DcP5 were examined in vitro and in vivo. The results demonstrated that P5 significantly inhibited the cell proliferation in FGFR2-dependent manner in DU145 cells (Fig. 1) and retarded tumor growth in DU145 xenograft model with negligible toxicity toward normal organs. Further investigations found that the Gln4 and Glu6 residues of P5 bind to FGFR2 to abolish its activation. Moreover, the authors developed the P5 cyclic derivative, DcP5, which achieved reinforced stability and anticancer activity in vivo. Their findings suggest P5 and its cyclic derivative DcP5 as potential candidates for anticancer therapy.
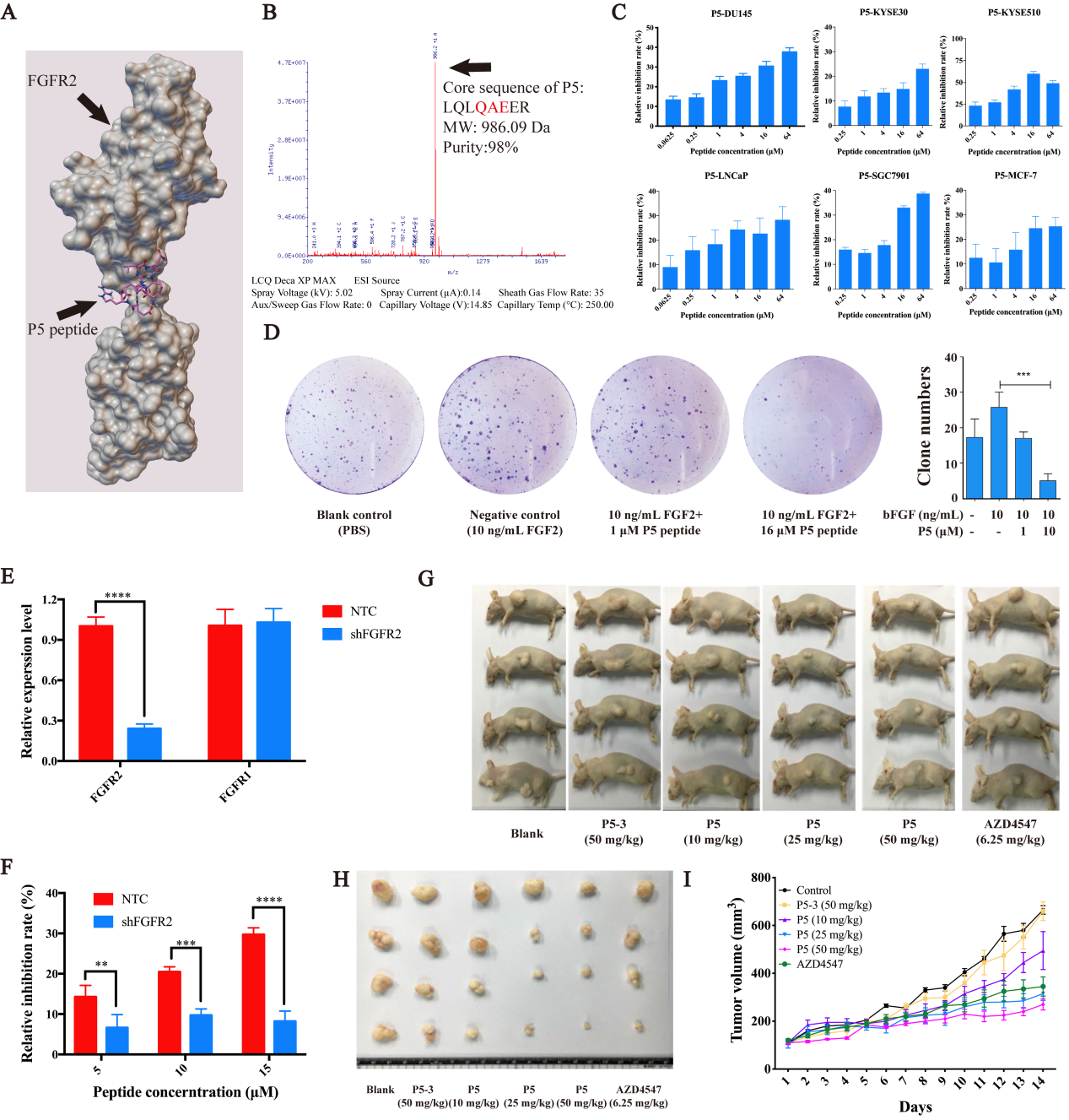
Fig. 1 Peptide design and the anticancer activities of P5 in vitro and in vivo
Article Access: https://onlinelibrary.wiley.com/doi/full/10.1002/mco2.48
Website for MedComm: https://onlinelibrary.wiley.com/journal/26882663
Looking forward to your contributions.


